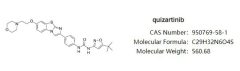
Daiichi Sankyo’s new drug for the treatment of AML receives FDA priority channel
On October 24, the Japanese pharmaceutical company Daiichi Sankyo announced that the U.S. Food and Drug Administration (FDA) has accepted the New Drug Application (NDA)

On October 24, the Japanese pharmaceutical company Daiichi Sankyo announced that the U.S. Food and Drug Administration (FDA) has accepted the New Drug Application (NDA)
On August 23, Daiichi Sankyo announced that the European Medicines Agency (EMA) has completed the marketing authorization validation of Quizartinib for the treatment of adults