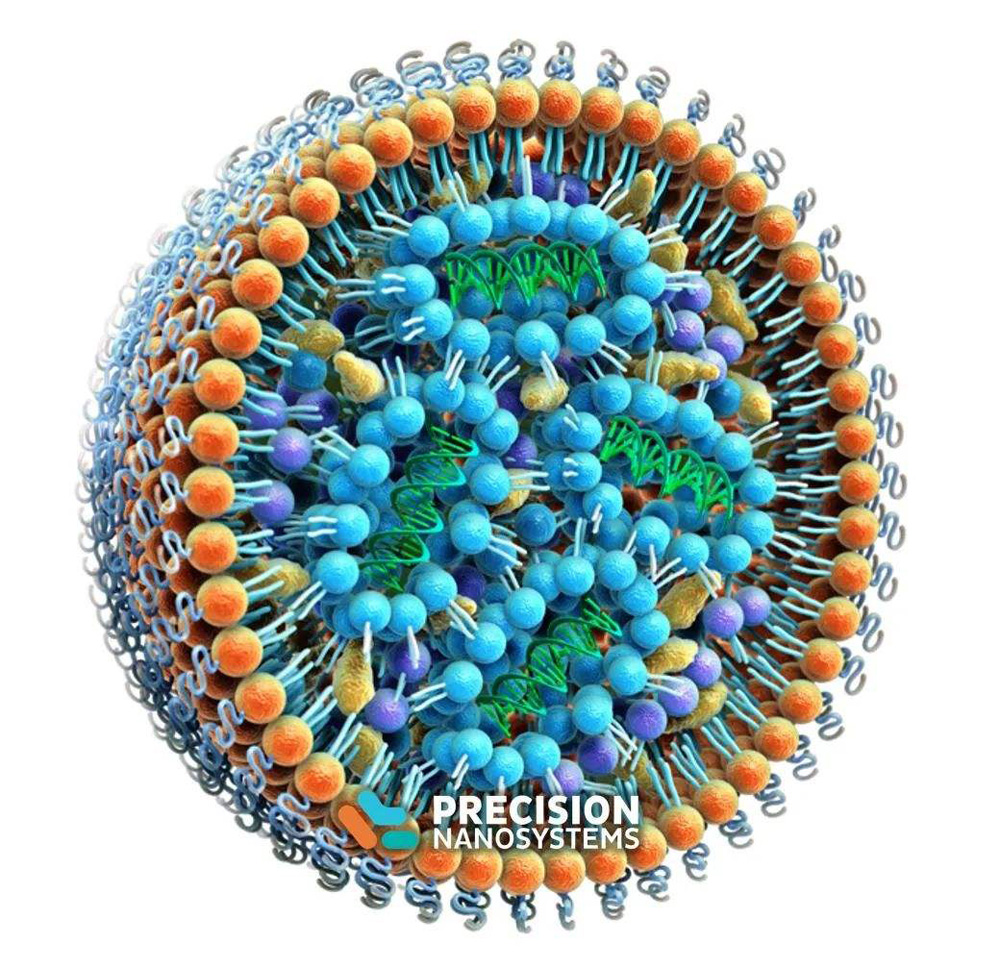
As an important component of current COVID-19 mRNA vaccines, lipid nanoparticles (LNPs) play a key role in effectively protecting mRNA and transporting it to cells. Liposomes, an early version of LNPs, are multifunctional nanodrug delivery platforms.
Toxicity of lipids in lipid nanoparticles
The constituent components of lipid nanoparticles are considered to be pharmacologically inactive and less toxic. However, in some cases, lipid nanoparticles are not non-immunogenic and certain unnatural compounds may be cytotoxic to humans, for example, although cationic lipids have broad potential as carriers for the delivery of fragile drugs such as nucleic acids Promising, but some cationic lipids can cause cytotoxicity.
Cationic lipids reduce cellular mitosis under certain circumstances, form vacuoles in the cytoplasm, and cause deleterious effects on key cellular proteins such as protein kinase C. The cytotoxicity of cationic lipids depends on the structure of their hydrophilic head groups; lipids with quaternary ammonium head groups are more toxic than lipids with tertiary amine head groups.
The effect of hydrophobic chains on lipotoxicity has not been fully validated, which limits the design to reduce lipotoxicity. The hydrophobic chains of lipid molecules are involved in particle behavior and efficacy, but certain lipids are associated with membrane damage and cytotoxicity. PEG-lipid conjugates can also lead to undesirable toxicity, and nanoparticles containing PEG-lipid conjugates are known to interact with immune cells, producing undesirable antibodies against PEGylated lipids.
2. Preparation of lipid nanoparticles
Various techniques have been used to control the properties of lipid nanoparticles, including particle size, the number of concentric lipid layers (lamellarity), and their ability to encapsulate drugs.
Thin-film hydration is the simplest and most electrostatic method for preparing liposomes. The lipid is dissolved in an organic solvent, evaporated and dried to form a film on the bottom of the bottle, and the lipid film is hydrated in an aqueous medium to form a dispersed liposome. Hydration conditions can affect the structure of the resulting lipid vesicles: mild hydration results in the formation of large unilamellar vesicles (GUVs), while vigorous agitation results in the formation of multilamellar vesicles with uneven particle size (Multilamellar vesicles, MLV), probe ultrasound or water bath ultrasound can be used to control particle size to form small unilamellar vesicles (Small unilamellar vesicles, SUV). Continuous extrusion of polycarbonate filters of defined pore size can also be used for liposome particle size control; the number of cyclic extrusions has a significant effect on the uniformity of the formed liposomes.
Another traditional method for preparing liposomes is the reverse evaporation method, which involves emulsification between an aqueous phase and a lipid-containing organic phase to form a water-in-oil emulsion. The mixed emulsion is subjected to a short-time ultrasonic treatment to homogenize it, and the organic solvent is removed under reduced pressure to form a gel, and an aqueous medium is added for hydration to form a liposome suspension. Solvent injection methods for liposome formation include rapid injection of ethanol or ether lipid solutions into aqueous media for dispersion. Detergent liposome preparation techniques involve dissolving phospholipids in an aqueous solution containing detergent (to a critical micelle concentration), then removing the detergent by dialysis or other means, diluting the resulting suspension with an aqueous solution, and reconstituting to form micelles; over time, the micelles are converted into liposomes. In the thermal method for preparing liposomes, the lipid is hydrated and then heated above the transition temperature of the phospholipid in the presence of a hydrating agent such as glycerol or propylene glycol, which is attractive because no organic solvent is involved .
A recently developed technique for liposome preparation is microfluidic hydrodynamic mixing, in which an alcoholic solution of lipids is placed in a central channel to flow, surrounded by a coaxial cross-flowing aqueous phase. The interdiffusion of ethanol and water at the mixed ethanol/water interface leads to lipid precipitation and self-assembly to form liposomes. Other recently developed techniques for preparing liposomes include cross-flow injection and methods using supercritical fluids.
Similarly, the preparation of other types of lipid nanoparticles, such as SLN, NLC, and cubic liposomes, also includes various homogenization methods (high shear homogenization, hot or cold homogenization, high speed homogenization) , sonication and microfluidization. Among them, ultrasonic, extrusion and microfluidic methods are the most commonly used methods to control the particle size of lipid nanoparticles.
The composition of the three-type nanoparticles in the patent
Many components are used in lipid nanoparticles (LNPs), and the components determine the morphology and application of the nanoparticles. LNPs typically contain ionizable cationic lipids and PEG-lipid conjugates (PEG-lipids), among other components, in addition to using the most common phospholipids and cholesterol. There are about 45,000 patents related to LNP in the American Chemical Abstracts Service (CAS), and the application of each component is as follows:
Cholesterol (Cholesterol, CAS number: 57-88-5) is the most used lipid component in patents, and more than 3,200 LNP patents use formulations containing cholesterol.
Phospholipids are the most prevalent lipid class in LNP formulations. The most common phospholipids include phosphatidylcholines (PCs), phosphatidylethanolamines (PEs), phosphatidylglycerols (PGs), and phosphatidylserines (PSs). In terms of hydrocarbon chains of phospholipids, saturated dimyristoyl (14:0/14:0), dipalmitoyl (16:0/16:0) and distearoyl (18:0/18:0) alkanes are preferred chain and an unsaturated dioleoyl (18:1c9/18:1c9) alkane chain. Phospholipids of natural origin, such as total soybean phospholipids, soybean phosphatidylcholine, hydrogenated soybean phosphatidylcholine, and egg yolk lecithin are also frequently used in lipid nanoparticles.
PEG-lipid conjugates are conjugates of polyethylene glycol and lipids. PEGylated lipids have been widely used in lipid nanoparticles since the discovery that PEG-lipid conjugates can extend circulatory half-life in the form of “stealth” liposomes by increasing steric stabilization.
Cationic Lipids are usually various amine derivatives (such as DOGS and DC-Chol), quaternary ammonium compounds (such as DOTMA, DOTAP, DORIE and DMRIE), cationic phosphatidylcholines (such as EDOPC and EDMPC), amines Compositions of (such as DOSPA and GAP-DLRIE), amidine salts (such as Vitamidine). Cationic multi-charged head groups, such as DOSPA and DOGS, have been reported to be more effective than single-charged cationic lipids, such as DOTMA, DOTAP, DC-Chol, and DMRIE. The increase in potency may be related to the cohesion and nucleic acid-protecting ability of highly charged cationic lipids, but increasing the binding of the highly charged cationic lipids to nucleic acids may hinder or inhibit the release of nucleic acids within cells. In addition, the combination of quaternary ammonium salt and polyamine can significantly improve the delivery efficiency, the earliest cationic lipid Lipofectamine (CAS number: 158571-62-1) containing quaternary ammonium and polyamine moieties, by DOSPA: dioleoylphosphatidylethanolamine ( DOPE) according to a 3:1 mixture, is a highly efficient transfection agent.
References
Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement