Antibody-drug conjugates (ADCs) are the coupling of cytotoxic small-molecule drugs to targeted monoclonal antibodies through ADC linkers, which can reduce the toxicity of small-molecule drugs and increase the therapeutic window through the specificity of the antibodies.
Critical quality attributes
ADC drugs mainly have three components: 1. Specific targeting antibodies 2. High titer cytotoxic drugs 3. Linkers.
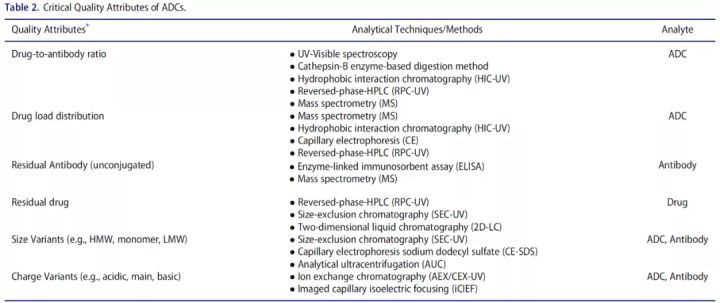
ADC drugs are structurally complex, highly heterogeneous, and contain multiple product-related impurities, making it critical to determine their critical quality attributes.
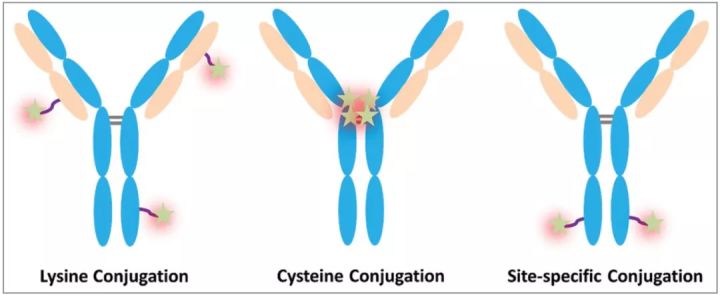
DAR (drug-to-antibody ratio)
DAR represents the average number of cytotoxic drugs conjugated to the antibody and is an important quality attribute that is directly related. Because low drug loading (low DAR) can reduce the potency of ADC, while high drug loading (high DAR) can alter the pharmacokinetics and toxicity of ADC molecules.
Controlling the conjugation reaction, especially reactant concentration, which results in DAR changes (especially in the case of random conjugation) is the most critical step in ADC development. Production and post-production handling/handling controls to reduce unconjugated naked antibody and low/high DAR products to keep DAR within target range.
From a product stability perspective, the degree of drug conjugation can alter the stability and aggregation propensity of the ADC.
Therefore, drug loading and drug distribution are CQAs that should be controlled in ADC generation. Spectroscopy, radiation, chromatography, mass spectrometry, etc. are commonly used analytical methods.
UV-Vis spectrometry
UV-Vis spectroscopy is a simple and most commonly used method for the determination of DAR, and the prerequisites for this method are: 1) The drug should contain UV-absorbing groups; 2) The drug and antibody exhibit distinctly independent absorption maxima in the UV-Vis spectrum 3) The presence of the drug should not affect the light absorption properties of the antibody in the ADC sample and vice versa.
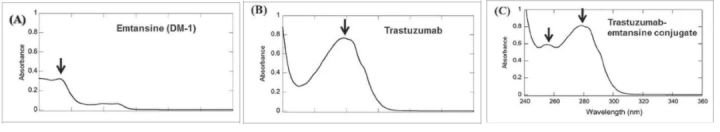
Determination of Trastuzumab emtansine conjugate by UV-Vis spectroscopy (Reference 3)
According to the measured absorbance and extinction coefficient, the concentration of protein and drug can be calculated according to the Beer–Lambert principle, and the average DAR can be calculated according to the following formula:
Mass spectrometry
Mass spectrometry is another commonly used method for analyzing DAR. Current uses include electrospray ionization mass spectrometry (ESI), time-of-flight mass spectrometry (TOF) or Orbitrap to distinguish heterogeneous molecular species of ADCs.
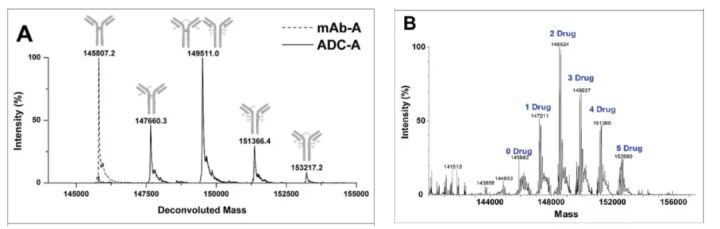
Determination of DAR by mass spectrometry According to the molecular weight and corresponding peak area determined by mass spectrometry, the average DAR is calculated.
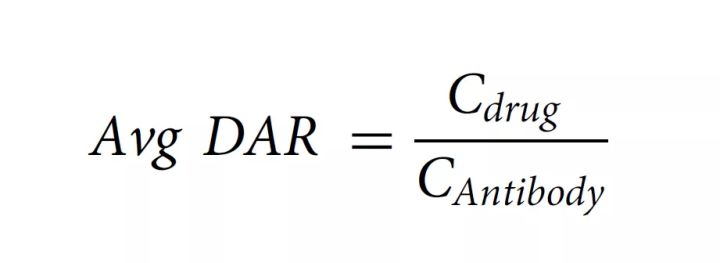
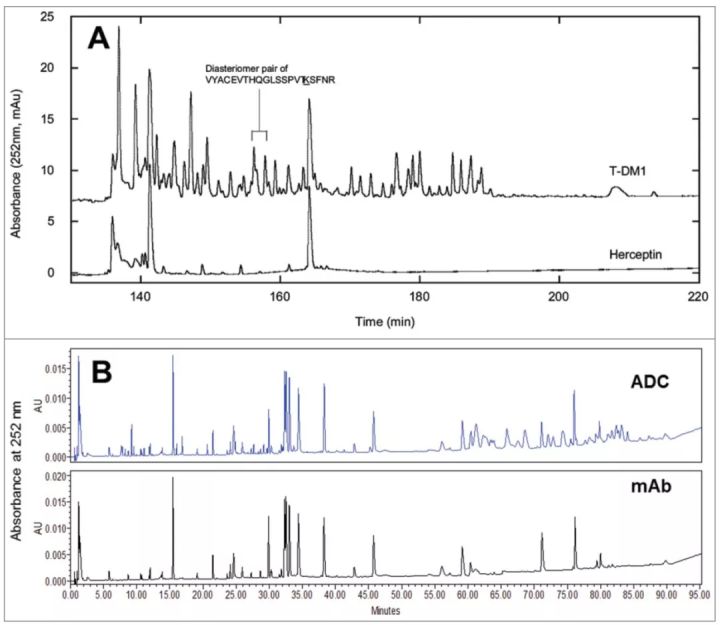
Drug load distribution
The overall drug loading distribution can be characterized by complete mass analysis, and less heterogeneous ADCs generated by cysteine or other site-specific coupling can also be analyzed by hydrophobic interaction chromatography. To determine the drug loading distribution at each conjugation site, a more comprehensive analysis, such as a peptide map, is required.
If a good chromatographic separation is achieved, accurate information about the occupancy of the coupling sites can be obtained with liquid chromatography. It should be pointed out that the conjugated compounds may affect the efficiency of proteolysis due to steric hindrance and conformational changes. Therefore, it is necessary to use a second enzyme in sequential or parallel processing to hydrolyze the ADC drug.
For ADCs with random conjugation, if the absorbance peaks of the conjugated drug and naked antibody do not overlap, the peptide map of the ADC can be used to compare and locate the peptides conjugated to the drug. The location of the peptide and the conjugation site to which the drug molecule is conjugated can be determined by LC-MS analysis.
The detection limit of this method is usually below 1%. When MS signals are used to quantify conjugated peptides, the effect of additional compounds on ionization efficiency due to loss of primary amine groups and introduction of hydrophobic moieties should be considered for accurate quantification. However, the effect of coupling on ionization efficiency may be less pronounced for intact proteins. Furthermore, trypsin/Lys-C, which is typically used for peptide mapping, does not cleave modified lysines, making it difficult to measure the occupancy of this site.
Another difficulty with ADC peptide mapping is the precipitation and adsorption of conjugated peptides on the surface of the assay vial, which can sometimes be alleviated by adding acetonitrile or isopropanol to the sample solution.
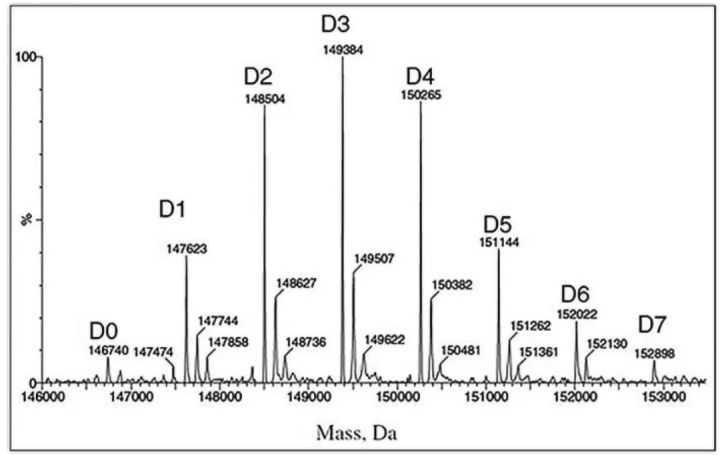
Unconjugated naked antibody
Naked antibodies compete with ADCs for target antigens, ultimately reducing the amount of drug delivered to target cells. The naked antibody level is thus a key parameter for process control and monitoring throughout the shelf life, as it directly affects the effectiveness of the drug.
Mass spectrometry is the most critical technical method for naked antibody analysis (as shown in the figure below, D0 is naked antibody). The percentage of bare resistance can be calculated from the area under the curve and the total area. Overall, LC-MS is the best method to fully characterize ADCs.
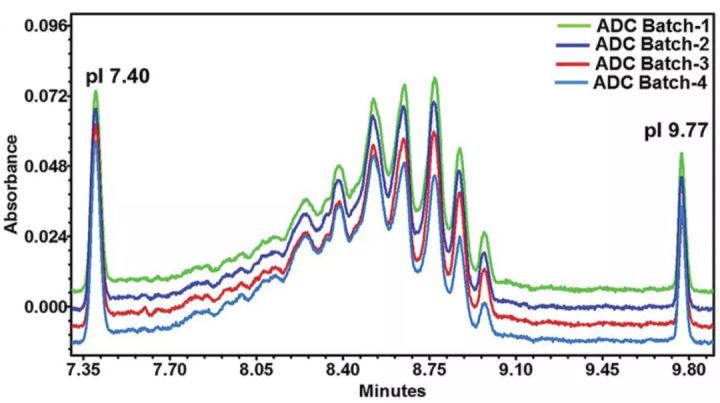
Imaging capillary electrophoresis (iCIEF) has also been used to analyze ADC drug loading distribution in recent years, but in general it faces a series of problems. Because the linker without drug coupling also causes the charge to shift toward the acid end, the analytical value of the drug loading distribution is higher than the actual value. In addition, iCIEF cannot distinguish between conjugates, process intermediates and impurities, such as antibodies with linker only and antibodies with linker/drug conjugates. Furthermore, iCIEF is not suitable for some conjugations such as cysteine, polysaccharides or site-specific conjugation chemistries, as they do not result in significant changes in charge and net pI.
Charge variant
Charge variation in protein therapeutics is an important mass property with potential effects on stability and biological activity. Various forms of charge-related variants can be observed during protein expression, drug conjugation, purification processes, and various post-translational modifications, including enzymatic or chemical degradation. IEX and capillary electrophoresis (CIEF and iCIEF) are commonly used detection methods, but neither works well for lysine-conjugated ADC drugs.
Molecular weight variants
Molecular weight size variation (polymers, particles, fragments) can directly affect the efficacy or safety of protein therapeutics. When two or more monomers aggregate, aggregates ranging in size from tens of nanometers to hundreds of micrometers are formed. Aggregation is the most common route of physical degradation of proteins. ADCs aggregate more easily than parent antibodies, mainly due to the presence of the drug/linker, which is more hydrophobic around the conjugation site. In addition, drug conjugation may also have a direct effect on the overall surface charge as well as the thermal stability of the protein.
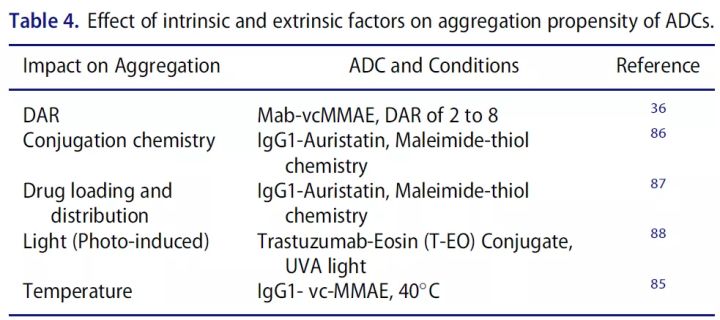
Fragmentation is the result of cleavage of peptide bonds by chemical or enzymatic reactions. Although protein backbones have relatively high stability, certain sites may be prone to fragmentation and concomitant fragmentation during aggregate formation.
Size exclusion chromatography and CE-SDS are commonly used to analyze molecular weight size variants, aggregates, etc.
In the past two years, several ADC drugs have been successfully approved, making them an important part of the pipeline of more and more drug R&D companies. ADCs combine the advantages of the specificity of antibody drugs and the cytotoxicity of small molecule drugs, increasing the therapeutic window. However, the coupling of the two also changes the physical and chemical properties of each other, causing changes in structure and charge. Compared with monoclonal antibody drugs, ADC drugs need to increase the consideration of quality attribute changes caused by conjugation, such as DAR, drug loading, naked antibody ratio, easy aggregation, etc.
references
1. Chen Y. Drug-to-antibody ratio (DAR) by UV/Vis spectroscopy. Methods Mol Biol. 2013;1045:267–73.
2. Adem YT, et al, Antibody Drug Conjugate Physical Instability and the Role of Drug Payload. Bioconjugate Chemistry. 2014;25:656–64.
3. Anil Wagh et al, Challenges and new frontiers in analytical characterization of antibody-drug conjugates, MABS 2018, VOL. 10, NO. 2, 222–243
4. Jinsha Liu et al, Therapeutic Advances in Oncology, Int. J. Mol. Sci. 2021, 22, 2008.