Recently, Arcutis Biotherapeutics announced that the FDA approved the topical selective PDE4 inhibitor ZORYVE (Roflumilast, roflumilast) cream 0.3% for the treatment of plaque psoriasis in patients 12 years and older. ZORYVE is the world’s first and only topical PDE4 inhibitor approved for the treatment of plaque psoriasis, which rapidly clears psoriatic plaques and relieves itching in all affected areas.
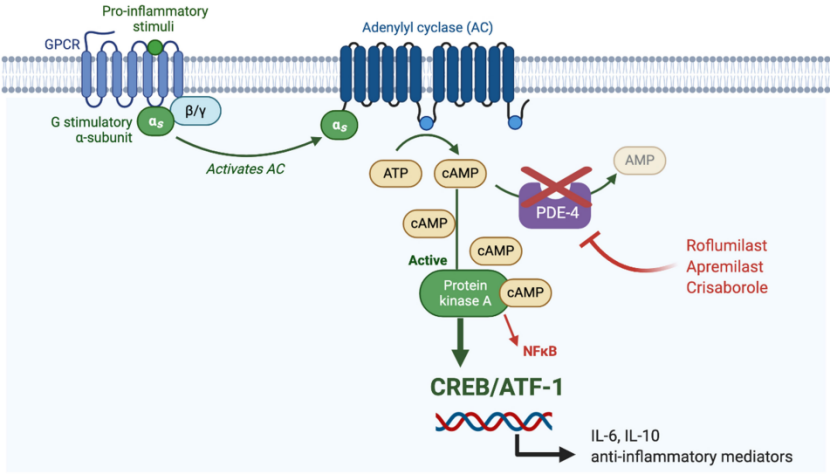
Phosphodiesterase 4 (PDE-4) can degrade its substrate cyclic adenosine monophosphate (cAMP) to adenosine monophosphate (AMP), thereby mediating the production of pro-inflammatory mediators and playing a role in the pathogenesis of psoriasis. plays a vital role. PDE-4 inhibitors can inhibit the occurrence of inflammation by blocking the degradation of cAMP.
This FDA approval is based on the combined results of two pivotal Phase III clinical trials, DERMIS-1 and DERMIS-2. DERMIS-1 and DERMIS-2 are randomized, parallel, double-blind, vehicle-controlled, multinational, multicenter Phase III studies to evaluate the efficacy and safety of roflumilast cream 0.3% in patients with plaque psoriasis sex.
The results of the study showed that at week eight, patients treated with roflumilast cream 0.3% had significantly increased success rates on the Investigator’s Global Assessment (IGA) compared to the control group (DERMIS-1 treatment). 42% in the DERMIS-2 arm and 37% in the DERMIS-2 arm; compared to 6% in the DERMIS-1 arm and 7% in the DERMIS-2 arm). IGA success was defined as psoriasis eradicated (IGA score of 0), nearly eradicated (IGA score of 1), and an improvement of at least two grades from baseline in IGA score. Meanwhile, ZORYVE demonstrated a favorable safety and tolerability profile in both trials.
“Arcutis has reached an important milestone, bringing a new generation of topical PDE4 inhibitors to adults and adolescents with plaque psoriasis,” said Frank Watanabe, President and CEO of Arcutis. As a result, we are delighted to launch ZORYVE, which is expected to be available in mid-August.”