On October 26, ESSA Pharma announced the latest positive results from the first two cohorts of the company’s lead drug candidate EPI-7386 in combination with enzalutamide (enzalutamide) in patients with metastatic castration-resistant prostate cancer (mCRPC) in a Phase I/II study clinical data.
Affected by this news, ESSA’s share price rose 180%.
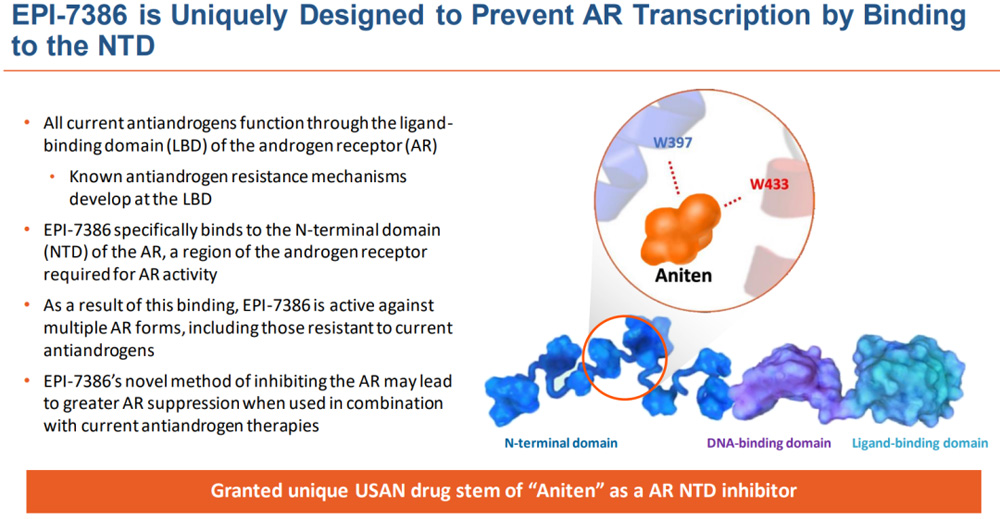
EPI-7386 is a highly selective oral androgen receptor N-terminal domain (AR NTD) small molecule inhibitor. All current antiandrogens act through the ligand-binding domain (LBD) of the androgen receptor (AR). In contrast, EPI-7386 specifically binds to the N-terminal domain (NTD) of the androgen receptor domain required for AR activation. Thus, EPI-7386 is active against multiple AR forms, including those that are resistant to current antiandrogens. This new approach to inhibiting AR in combination with current antiandrogen therapy may result in stronger AR inhibition.
EPI-7386 is currently in a Phase I clinical trial for patients with castrated prostate cancer (CRPC) whose tumors have progressed on standard therapy. The U.S. Food and Drug Administration granted Fast Track designation to EPI-7386 for the treatment of adult male mCRPC patients who are resistant to standard therapy. ESSA is also conducting a Phase I/II clinical trial of EPI-7386 in combination with enzalutamide for metast.
In this multicenter, open-label, phase I/II dose-escalation study, seven patients with mCRPC who had not received second-generation antiandrogen therapy were enrolled in the first two cohorts, receiving EPI-7386 escalating doses and enzalutamide A fixed dose of 120 mg (QD) once daily.
The results showed that the safety of the combination therapy was good, consistent with second-generation antiandrogens, and no dose-limiting toxicity was observed. Efficacy could not be assessed in one patient in one cohort who discontinued after one treatment cycle because of a strong CYP3A inducer that reduced EPI-7386 and enzalutamide exposure. The antitumor activity of the remaining 6 patients showed that after 12 weeks of treatment, 4 out of 6 patients achieved PSA90 remission, and 5 out of 6 patients achieved PSA90 remission so far.
Pharmacokinetic results from the first two cohorts showed that EPI-7386 had little effect on enzalutamide exposure, while the combination resulted in a reduction in EPI-7386 exposure, which remained within the clinically relevant range suggested by studies in preclinical xenograft models Inside.
“We are encouraged by the rapid and deep PSA remission observed in the dose-escalation study of EPI-7386 in combination with enzalutamide,” said Dr. David Parkinson, ESSA President and CEO. “This therapy is safe and well-tolerated. The kinetic results suggest that EPI-7386 has little effect on enzalutamide exposure. We are currently recruiting a third dose-escalation cohort to optimize the therapeutic dose of EPI-7386 in combination therapy in preparation for a Phase II trial.