Hemophilia gene therapy priced at $2.9 million
On November 3, the Institute of Clinical and Economic Review (ICER) announced that, after a cost-benefit analysis, it assessed the price ceiling of Roctavian (valoctocogene

On November 3, the Institute of Clinical and Economic Review (ICER) announced that, after a cost-benefit analysis, it assessed the price ceiling of Roctavian (valoctocogene

Ascendis Pharma announced that the FDA has accepted and granted Priority Review status for TransCon PTH for the treatment of adult patients with hypoparathyroidism, with

On November 1, Rhythm Pharmaceuticals announced that its MC4R agonist setmelanotide was granted Breakthrough Therapy Designation by the FDA for the treatment of hypothalamic obesity.
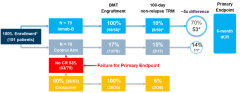
On October 31, Actinium Pharmaceuticals announced that the pivotal Phase III SIERRA study of its CD45 monoclonal antibody Iomab-B (apamistamab) in patients with relapsed or
On October 31, ESSA Pharma announced that Johnson & Johnson/Janssen Pharmaceuticals will discontinue a Phase I combination therapy clinical study of its lead candidate, EPI-7386,

Minerva Neurosciences is a biopharmaceutical company focused on developing therapies for diseases of the central nervous system (CNS). Recently, the company announced that it received
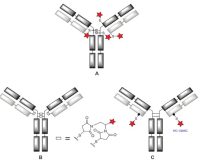
The conjugation of Linker-drug (LD) to antibodies is a critical step in the generation of ADCs and the determination of key quality attributes of ADCs

On October 27, Gilead announced its Q3 financial report and revealed that its marketing application for bulevirtide (trade name: Hepcludex) for the treatment of chronic

On October 27, Merck announced its third-quarter financial report, and its product pipeline no longer includes the oncolytic virus therapy Cavatak (V937) obtained from the
Gilead Sciences recently announced the real-world data (RWD) of the three-in-one compound new drug Biktarvy BICSTaR study at the 30th International Conference on Drug Therapy
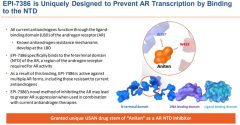
On October 26, ESSA Pharma announced the latest positive results from the first two cohorts of the company’s lead drug candidate EPI-7386 in combination with
Eisai recently announced the final results of a real-world data (RWD) study at the 91st American Thyroid Association (ATA) Annual Meeting in 2022. This study