ADC Therapeutics: Completed first patient dosing of Zynlonta in Phase 1b LOTIS-7 trial
On June 29, 2022, ADC Therapeutics announced that it has completed the first patient dosing of Zynlonta in the Phase 1b LOTIS-7 trial evaluating combination

On June 29, 2022, ADC Therapeutics announced that it has completed the first patient dosing of Zynlonta in the Phase 1b LOTIS-7 trial evaluating combination
Cancer cells are malignant tumor cells derived from epithelial cells, which have the characteristics of infinite proliferation, transformation and easy transfer. Recently, scientists have found

Breast cancer is the most common cancer and one of the leading causes of cancer-related deaths worldwide. In 2020, more than 2 million cases of

I. INTRODUCTION This guidance provides recommendations to assist industry in the development of oligonucleotide therapeutics under section 505 of the Federal Food, Drug, and Cosmetic
On June 23, Xinghand Bio announced that the company first announced its targeting hepatitis B virus surface antigen (HBsAg) at the 57th European Association for
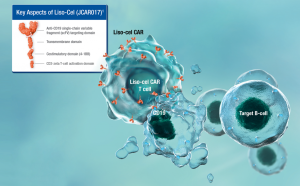
On June 24, BMS announced that the FDA approved a new indication of the CD19 CAR-T therapy Breyanzi (lisocabtagene maraleucel) for the second-line treatment of

On June 22, several Japanese media, including the Mainichi Shimbun, reported that Shionogi’s S-217622 emergency authorization application based on Phase II data was suspended by
On June 22, Merck announced that Erbitux® (cetuximab injection) was officially approved by the China National Medical Products Administration (NMPA) for the treatment of locally
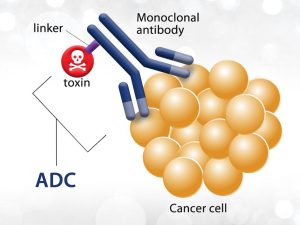
ADC (antibody-drug conjugate) has become one of the research hotspots in the field of oncology in recent years. At the 2022 American Society of Clinical