Cellectis: FDA Approves IND Application for UCART20x22
On August 1, Universal CAR-T (UCAR-T) pioneer Cellectis announced that the U.S. FDA has approved the company’s IND application for UCART20x22 to initiate this product

On August 1, Universal CAR-T (UCAR-T) pioneer Cellectis announced that the U.S. FDA has approved the company’s IND application for UCART20x22 to initiate this product

French pharmaceutical group Sanofi announced on Thursday that it will partner with Chinese biotech company Innovent Biologics to develop anti-cancer drugs for the Chinese market.

On August 1, Johnson & Johnson/Janssen announced that the FDA approved an expanded indication of ustekinumab (Stelara) for the treatment of pediatric patients 6 years

On August 1, 2022, base editing company Beam Therapeutics announced that its IND application for BEAM-201 was suspended by the FDA. The FDA will provide
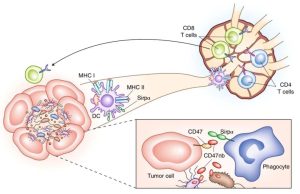
ALX Oncology’s Evorpacept Receives Fast Track Designation from FDA as First-Line Treatment for Head and Neck Squamous Cell Carcinoma ALX Oncology On August 1, ALX
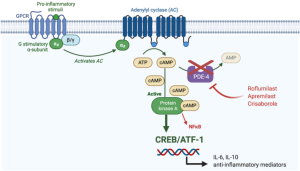
Recently, Arcutis Biotherapeutics announced that the FDA approved the topical selective PDE4 inhibitor ZORYVE (Roflumilast, roflumilast) cream 0.3% for the treatment of plaque psoriasis in
On July 29, AbbVie announced that the European Commission (EC) has approved upatinib (15 mg once daily) for the treatment of adults with active non-radiographic

Sarepta Therapeutics Announces Intent to Submit an Accelerated Approval Biologics License Application for its Gene Therapy SRP-9001 to Treat Duchenne Muscular Dystrophy Sarepta On July
Recently, Editas Medicine announced data from the Phase I/II RUBY trial of EDIT-301 therapy for the treatment of sickle cell anemia (SCD), with the first
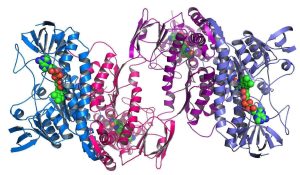
‘The entire protein universe’: AI predicts shape of nearly every known protein DeepMind’s AlphaFold tool has determined the structures of around 200 million proteins. Nature

FDA Accepts Biogen’s New Drug Application and Grants Priority Review of Tofersen for a Rare, Genetic Form of ALSCAMBRIDGE, Mass., July 26, 2022 (GLOBE NEWSWIRE)
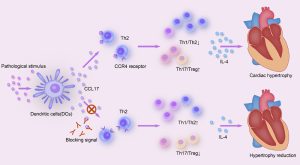
Pathological cardiac remodeling characterized by left ventricular hypertrophy, myocardial fibrosis, and inflammation is a determinant of the clinical course of heart failure (HF). Aging and