RWD study: Lenvima shows high efficacy in thyroid cancer
Eisai recently announced the final results of a real-world data (RWD) study at the 91st American Thyroid Association (ATA) Annual Meeting in 2022. This study

Eisai recently announced the final results of a real-world data (RWD) study at the 91st American Thyroid Association (ATA) Annual Meeting in 2022. This study

On October 26, AstraZeneca announced that the Phase III CAPItello-291 study of its Akt1/2/3 inhibitor capivasertib in combination with fulvestrant in breast cancer patients met
With uptake slow in the United States for COVID-19 booster shots that the government supplies for free, what will a 400% price increase do to

On October 24, Alpine Immune Sciences announced that it had terminated two clinical trials of the conditional CD28 costimulator and dual PD-L1/CTLA-4 checkpoint inhibitor davoceticept
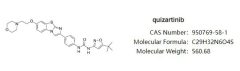
On October 24, the Japanese pharmaceutical company Daiichi Sankyo announced that the U.S. Food and Drug Administration (FDA) has accepted the New Drug Application (NDA)

On October 24, Tricida announced key results from the Phase III VALOR-CKD clinical trial, which was designed to evaluate the ability of veverimer to slow
On October 20, Talaris announced the latest progress of the Phase III FREEDOM-1 study of its investigational allogeneic cell therapy FCR001 for inhibiting acute immune
On October 21, the FDA’s official website showed that AstraZeneca’s anti-CTLA-4 monoclonal antibody tremelimumab (temslimumab) was approved by the FDA for listing, and the approved
The results of a new study show that a bispecific antibody drug, Ubamatamab, has shown good results in ovarian cancer patients and has a good

On October 20, Immunic announced that its Phase Ib clinical trial of its RORγt inverse agonist IMU-935 in the treatment of psoriasis failed to meet

On October 19, GSK announced that the ZOSTER-049 extension study yielded positive interim results: Shingrix provides at least 10 years of shingles protection in adults

On October 20, Roche and HookipaPharma entered into a strategic collaboration and licensing agreement to co-develop HB-700 and a second undisclosed novel arenavirus immunotherapy for