IND application for BEAM-201 suspended by FDA
On August 1, 2022, base editing company Beam Therapeutics announced that its IND application for BEAM-201 was suspended by the FDA. The FDA will provide

On August 1, 2022, base editing company Beam Therapeutics announced that its IND application for BEAM-201 was suspended by the FDA. The FDA will provide
ALX Oncology’s Evorpacept Receives Fast Track Designation from FDA as First-Line Treatment for Head and Neck Squamous Cell Carcinoma ALX Oncology On August 1, ALX
Antibodies are secreted by plasma cells transformed from B lymphocytes, and each B lymphocyte strain can only produce one type of antibody specific to one
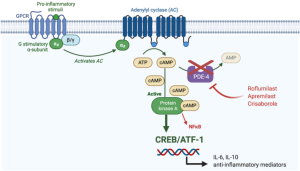
Recently, Arcutis Biotherapeutics announced that the FDA approved the topical selective PDE4 inhibitor ZORYVE (Roflumilast, roflumilast) cream 0.3% for the treatment of plaque psoriasis in
On July 29, AbbVie announced that the European Commission (EC) has approved upatinib (15 mg once daily) for the treatment of adults with active non-radiographic

Sarepta Therapeutics Announces Intent to Submit an Accelerated Approval Biologics License Application for its Gene Therapy SRP-9001 to Treat Duchenne Muscular Dystrophy Sarepta On July
Recently, Editas Medicine announced data from the Phase I/II RUBY trial of EDIT-301 therapy for the treatment of sickle cell anemia (SCD), with the first
‘The entire protein universe’: AI predicts shape of nearly every known protein DeepMind’s AlphaFold tool has determined the structures of around 200 million proteins. Nature

FDA Accepts Biogen’s New Drug Application and Grants Priority Review of Tofersen for a Rare, Genetic Form of ALSCAMBRIDGE, Mass., July 26, 2022 (GLOBE NEWSWIRE)
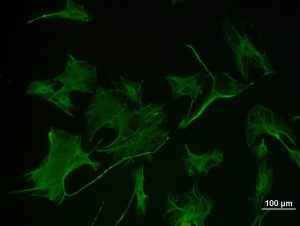
A fluorophore is a fluorescent dye that acts as a fluorescent marker for protein, tissue, and cell labeling for detection by fluorescence microscopy. Fluorophores work
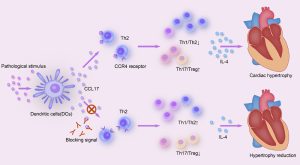
Pathological cardiac remodeling characterized by left ventricular hypertrophy, myocardial fibrosis, and inflammation is a determinant of the clinical course of heart failure (HF). Aging and
On July 26, Brooklyn announced results from the Phase II INSPIRE trial of IRX-2, a multi-cytokine biological immunotherapy. The trial was designed to evaluate the