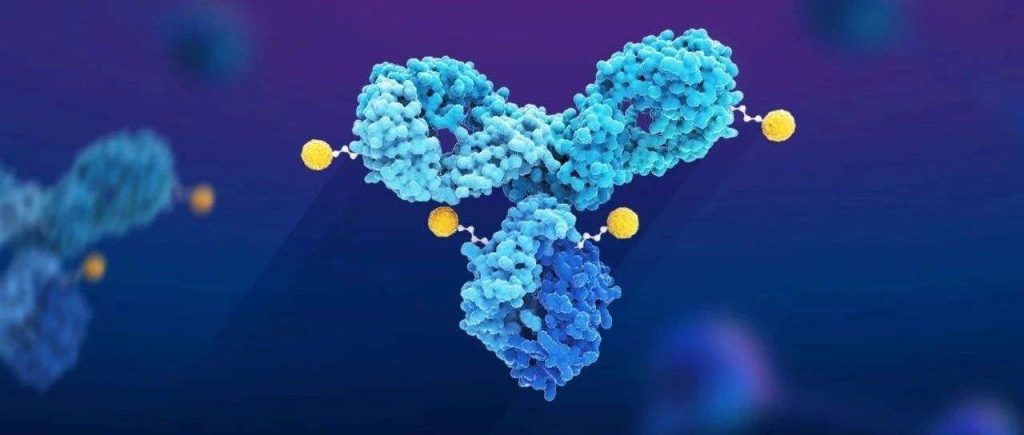
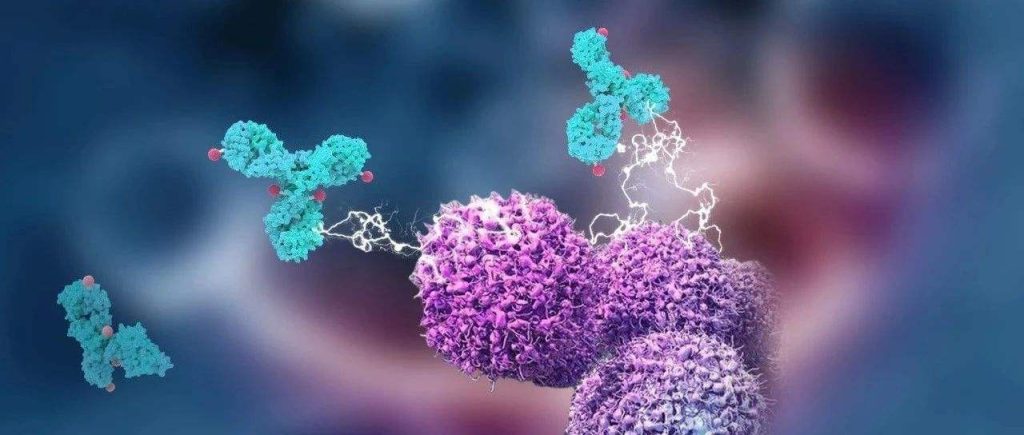
Introduction of ADC Bioconjugation Technology
The design of clinically successful ADCs depends not only on the potency of the payload and its point of attachment, linker stability, and efficient drug
The design of clinically successful ADCs depends not only on the potency of the payload and its point of attachment, linker stability, and efficient drug

The two sister disciplines of clinical pharmacology and clinical pharmacy were newly emerging disciplines in the 1960s. Penetration between peripheral disciplines is particularly prominent among

Aktis Oncology, a biotechnology company focused on the discovery and development of novel targeted alpha radiopharmaceuticals to treat solid tumors, announced the completion of an
On August 27, AstraZeneca updated the analysis results of the Phase III DAPA-HF and DELIVER studies, compared with placebo, dapagliflozin (brand name: Farxiga) can significantly
Alcoholic liver disease (ALD) is a common chronic liver disease that often leads to cirrhosis. With the rising incidence, alcoholic liver disease has become the
Moderna Sues Pfizer and BioNTech Over Covid Vaccine Technology Two lawsuits filed in Massachusetts and Germany claim the Pfizer-BioNTech Covid-19 vaccine violated Moderna’s mRNA patents.

1. Q: What should I do if I want to reuse the ultrafiltration tube provided in the coupling kit?answer:Use a pipette tip to absorb a

According to Bloomberg, citing people familiar with the matter, the two companies have so far failed to agree on a price in talks to acquire
On August 24, BioMarin announced that the European Union approved the conditional marketing of Roctavian (Valoctocogene roxaparvovec) gene therapy for the treatment of adults with

On August 24, the “New England Journal of Medicine” published a real-world data of Pfizer’s new crown oral drug Paxlovid (nirmatrelvir + ritonavir) against Omicron
On August 23, Merck announced that the FDA has granted Fast Track designation to its intravenous anticoagulant drug MK-2060 for reducing the risk of major
On August 23, Daiichi Sankyo announced that the European Medicines Agency (EMA) has completed the marketing authorization validation of Quizartinib for the treatment of adults