MC2-25-C1 new drug for uremic pruritus starts phase II clinical trial
On September 14, MC2 Therapeutics announced that it had completed the first patient dosing of the Phase II MC2-25-C1 trial. The trial was designed to

On September 14, MC2 Therapeutics announced that it had completed the first patient dosing of the Phase II MC2-25-C1 trial. The trial was designed to

According to the official website of the World Health Organization, on September 14, WHO Director-General Tedros Adhanom Ghebreyesus said at an online press conference that
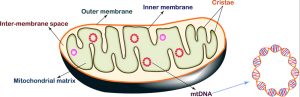
On September 12, Pretzel Therapeutics, a biotechnology company, announced the completion of a $72.5 million Series A financing to pioneer innovative therapies that modulate mitochondrial
On Sept. 13, Rubius Therapeutics announced plans to restructure its operations and implement a series of cost-saving measures in order to advance its next-generation red

On September 12, Nimbus Therapeutics announced the completion of a $125 million private placement financing. Participating in this round of financing includes two new investors
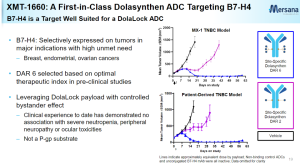
On September 12, Mersana announced that its ADC product XMT-1660 targeting was granted Fast Track designation by the FDA for the treatment of adult patients
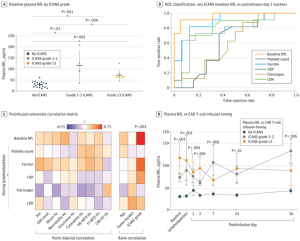
Cell-based immunotherapy — CAR-T cell therapy — has revolutionized the treatment of several cancers. The therapy uses genetically engineered T cells to target and attack
Targeted drugs are the most eye-catching progress in lung cancer treatment in recent years, which have significantly improved the prognosis of patients with advanced lung
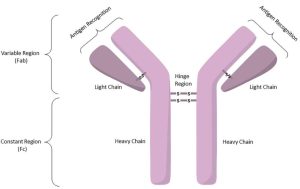
An ADC consists of three basic elements: a tumor-specific monoclonal antibody (mAb), a small cytotoxic molecule called a payload, and a linker (Linker) that connects
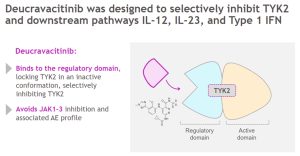
On September 10, Bristol-Myers Squibb (BMS) announced that the marketing application of its TYK2 inhibitor deucravacitinib for the treatment of plaque psoriasis has been approved
On September 7, Pfizer announced that its GBS vaccine candidate GBS6 (PF-06760805) has been granted breakthrough therapy designation by the FDA for the prevention of
Since the beginning of May this year, monkeypox cases have been reported in non-endemic countries, and monkeypox cases have continued to appear in several endemic