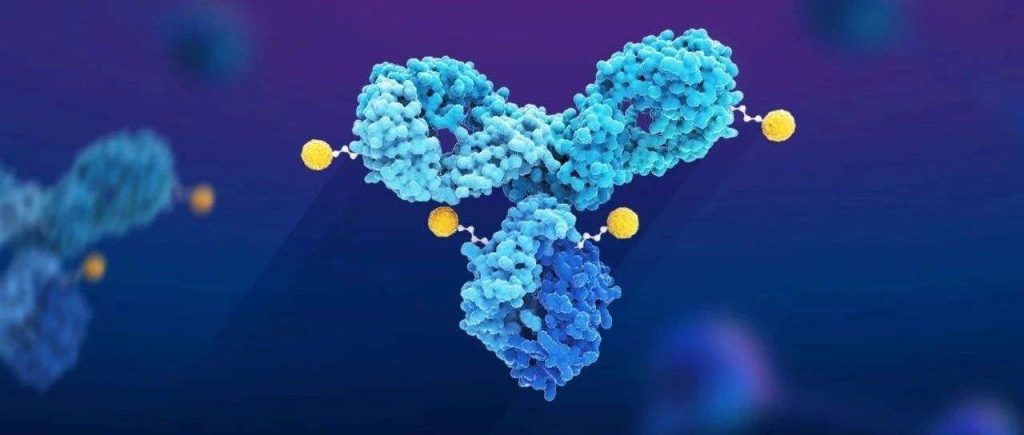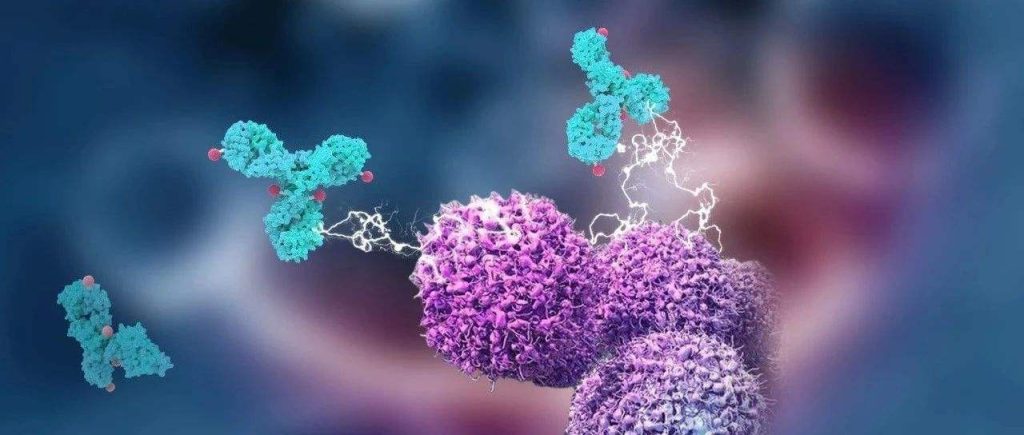
CAR-T cell therapy targeting CD19 is expected to treat lupus erythematosus
While there is no cure for lupus erythematosus and existing treatments don’t work for many of the 1.5 million lupus erythematosus patients in the U.S.,

While there is no cure for lupus erythematosus and existing treatments don’t work for many of the 1.5 million lupus erythematosus patients in the U.S.,
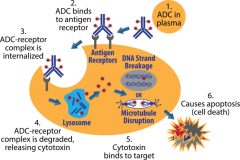
ADC Drug Assessment Background Before designing a DDI assessment strategy for a drug, an understanding of the properties of the drug itself is required. Therefore,
On September 20, Merck announced that it would initiate a new Phase III clinical trial designed to evaluate once-daily oral administration of Islatravir (100 mg)

On September 20, Cidara/Melinta Therapeutics jointly announced that the FDA has accepted the New Drug Application (NDA) for rezafungin (rezafungin) for the treatment of candidemia

On September 19, 2022, Pfizer announced that the U.S. Food and Drug Administration (FDA) has accepted the New Drug Application (NDA) for the oral JAK3/TEC
The immune system has evolved to protect the body from a variety of potential threats. These threats include bacterial diseases, such as plague, cholera, diphtheria,

On September 19, Roche announced that the marketing application of the ophthalmic dual anti-Faricimab (Vabysmo) has been approved by the European Union for the treatment

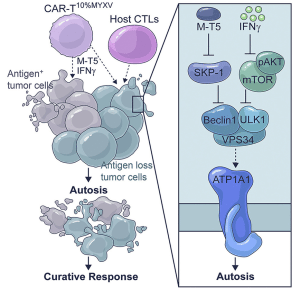
Cancer is unpredictable. The tumor shrinks after the initial treatment, but it can become more terrifying afterward. The disease can spread or mutate, and genetic
The rise of immunotherapy has greatly improved the treatment pattern of advanced lung cancer and provided more possibilities for personalized diagnosis and treatment of tumors.
In 2022, Zeng’s popular pathogen metagenomic sequencing (mNGS) has encountered unprecedented challenges. The emergence of pathogen-targeted sequencing (targeted NGS, tNGS) has made the market competition
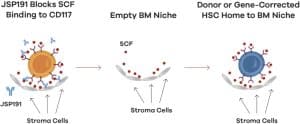
On September 15, Jasper Therapeutics announced that its CD117 monoclonal antibody JSP191 has been granted Fast Track designation by the FDA for the treatment of
Huge advances in artificial intelligence (AI) mean that researchers can design completely original molecules in seconds instead of months. In June, South Korean regulators approved