Oral ibandronate sodium tablets launched in China
On September 29, the CDE official website showed that the marketing application for ibandronate sodium tablets (Bonviva) has been accepted. Ibandronate sodium is an anti-osteoporosis

On September 29, the CDE official website showed that the marketing application for ibandronate sodium tablets (Bonviva) has been accepted. Ibandronate sodium is an anti-osteoporosis

When it comes to cancer, everyone is very worried. The incidence and mortality of cancer worldwide are rising. According to statistics [1], the global cancer
Colorectal cancer (CRC) is one of the most common malignant tumors, and its prevalence ranks third among malignant tumors. Despite the success of new targeted

On September 27, Exicure announced plans to restructure the company and integrate resources to continue exploring strategic alternatives to maximize shareholder value. Exicure will cut

On September 21, 2022, Eli Lilly announced that the U.S. Food and Drug Administration (FDA) has accelerated approval of its precision oncology drug Retevmo (selpercatinib,
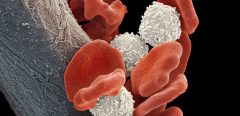
The oncogene EVI1 induces an aggressive leukemia, but the molecular mechanism behind it is still unclear; recently, a research report titled “EVI1 drives leukemogenesis through
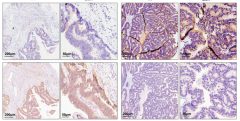
According to a recent GLOBOCAN, colorectal cancer (CRC) has the third highest incidence of all cancers and the second highest mortality rate in 2020, according
Multiple sclerosis is an autoimmune disease that causes damage to axons and neurons throughout the nervous system. While this damage is often unnoticed by patients

The Birth of ADC Drugs In today’s anti-tumor treatment, many chemotherapeutic drugs have been found to have a killing effect on tumor cells. Unfortunately, chemotherapeutic

In recent years, the ADC field has seen frequent blockbuster transactions and layouts, showing a pattern of contention among a hundred schools of thought. Only

Recently, a senior Republican committee aide revealed to the media Endpoints News that although policy negotiations including additional provisions (accelerated approval of reforms, etc.) are

According to the FDA’s official website, the indications of three PARP inhibitors for the last-line treatment of ovarian cancer have been withdrawn, namely AstraZeneca’s olaparib,