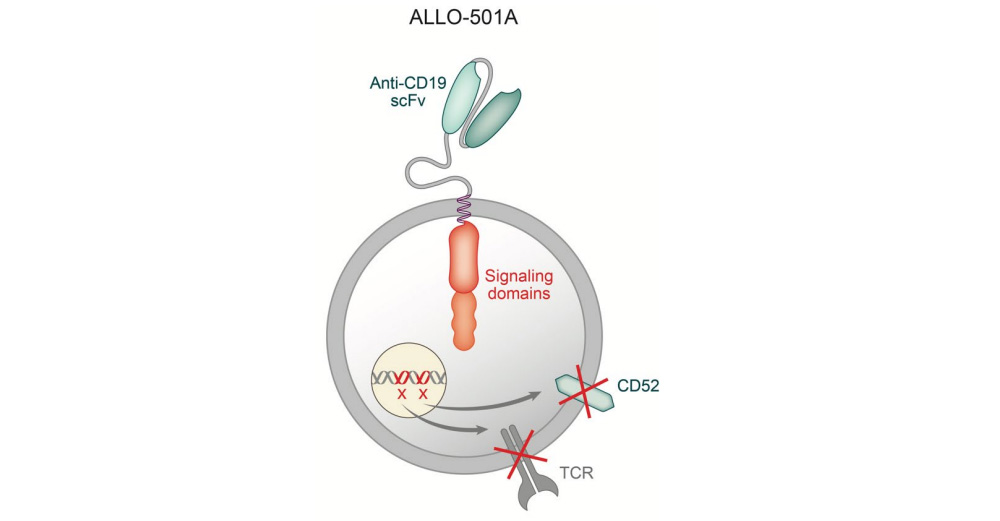
On October 6, Allogene announced the initiation of the Phase II ALPHA2 trial of the investigational allogeneic CAR T therapy ALLO-501A for the treatment of patients with relapsed/refractory large B-cell lymphoma (r/r LBCL).
ALLO-501A is an allogeneic CAR-T cell targeting CD19, knocking out the T cell receptor gene and CD52 gene through gene editing to reduce the risk of graft-versus-host disease (GvHD) and allogeneic rejection, while using Allogene The self-developed CD52 monoclonal antibody (ALLO-647) is used to suppress the host immune system to support the continuous treatment of CAR-T therapy and achieve sufficient efficacy. In June this year, the US FDA granted ALLO-501A Regenerative Medicine Advanced Therapy Designation (RMAT) for the treatment of r/r LBCL.
The single-arm Phase II ALPHA2 trial for the treatment of patients with r/r LBCL will use a single dose of ALLO-501A, including 120 million CAR T cells per dose. The ALPHA2 trial will enroll approximately 100 patients with r/r LBCL who have received at least two therapies and have not previously received anti-CD19 therapy. The primary endpoint of the trial will be objective response rate (ORR).
According to the Medicine Rubik’s Cube database, 8 autologous CAR T cell therapies have been approved for the market, including Yescarta (Aquilence) jointly developed by Kite Pharma/Fosun Kite and Carvykti (Carvykti) jointly developed by Tecartus and Legend Bio/Johnson & Johnson. Cidaki Orenza), Carteyva developed by WuXi Junuo, Kymirah developed by Novartis, liso-cel developed by BMS, Abecma jointly developed by Bluebird Bio/BMS/2seventy bio and ARI-0001 developed by Hospital Clínic de Barcelona.
In addition, the new drug marketing application for Iquilenxe injection jointly developed by Innovent Bio and Reindeer Bio has been accepted by CDE. This is the first domestically submitted new drug to be accepted for marketing and is expected to become the first approved BCMA-targeting autologous implant in China. Antigen receptor T cell immunotherapy products.
Autologous CAR-T cell therapy requires “private customization”, so there are limitations such as expensive production cost, long production time, and difficult to standardize the process. With the two advantages of “universal” and “off-the-shelf”, allogeneic immune cell therapy can significantly reduce the cost and time of patient treatment, which is called the next-generation revolution of cell therapy.
However, there are no allogeneic CAR-T drugs on the market yet. This type of CAR-T manufacturing process is easier, but there are several problems: allogeneic transplanted cells attack host cells and cause graft-versus-host disease (GvHD); Host-versus-graft reaction (HVGR) hinders the in vivo expansion of allogeneic cells and is less effective.
“We are proud to initiate the first Phase II trial of a potentially pivotal allogeneic CAR T product,” said Dr. David Chang, President and Co-Founder of Allogene. “This milestone is for ALLO-501A and our broader portfolio of innovative products. The line paves the way, and these products have the potential to greatly increase patients’ access to cell therapy.”
Allogene was founded in April 2018 by Arie Belldegrun, founder and CEO of Kite, and Dr. David Chang, the former executive vice president of R&D and chief medical officer of Kite. In December 2020, Allogene and Linglu Pharmaceutical announced the establishment of Allogene Overland Biopharm. The joint venture will focus on developing, manufacturing and commercializing Allo CAR-T therapies in Greater China, Taiwan, South Korea and Singapore.