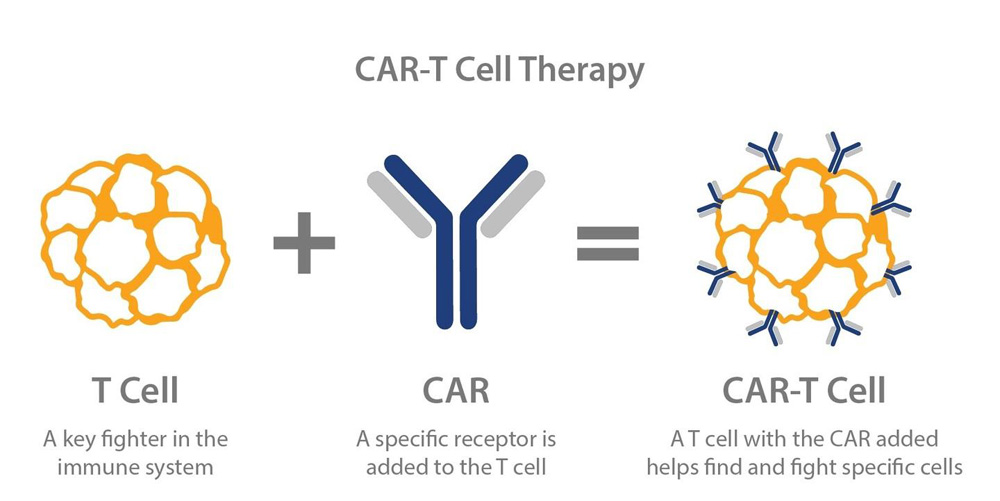
Chimeric Antigen Receptor T-cell (CAR-T) therapy is a type of cancer treatment that involves the genetic modification of a patient’s own T-cells, a type of immune cell, to recognize and target cancer cells. T-cells are a crucial part of the immune system, responsible for detecting and eliminating infected or abnormal cells in the body. However, cancer cells often evade detection by the immune system, allowing them to grow and multiply.
CAR-T therapy seeks to overcome this problem by altering the T-cells to recognize a specific protein, called an antigen, that is found on the surface of cancer cells. This is done by extracting T-cells from the patient’s blood and genetically modifying them to produce a chimeric antigen receptor (CAR) on their surface. The CAR is a protein that recognizes and binds to the cancer cell antigen, activating the T-cell to attack and kill the cancer cell.
The modified T-cells are then expanded in the laboratory and re-infused back into the patient, where they can target and eliminate cancer cells throughout the body. CAR-T therapy has been shown to be effective in treating certain types of cancer, including leukemia and lymphoma, and has the potential to be used for other types of cancer as well.
One of the main advantages of CAR-T therapy is its ability to specifically target cancer cells while leaving normal cells unharmed. This makes it an attractive alternative to traditional chemotherapy and radiation treatments, which often have significant side effects due to their non-specific nature. In addition, CAR-T therapy has the potential to provide long-lasting benefits, as the modified T-cells can continue to multiply and attack cancer cells for an extended period of time.
However, CAR-T therapy is not without its challenges and limitations. One of the main concerns is the potential for the modified T-cells to attack normal cells in the body, a side effect known as cytokine release syndrome (CRS). CRS can cause severe symptoms, including fever, difficulty breathing, and low blood pressure, and can be life-threatening in some cases. In addition, CAR-T therapy can also result in neurotoxicity, or damage to the nervous system, which can cause neurological symptoms such as headache, confusion, and seizures.
Another challenge is the high cost of CAR-T therapy, which can be upwards of $500,000 per patient. This has led to concerns about access to the treatment, as many patients may not be able to afford it. In addition, CAR-T therapy is currently only approved for use in certain types of cancer, and is not suitable for all patients.
Despite these challenges, CAR-T therapy represents a promising new approach to cancer treatment and has already had significant success in improving the outcomes of patients with certain types of cancer. Researchers are continuing to work on improving the safety and effectiveness of CAR-T therapy, as well as developing new CAR-T therapies for other types of cancer.